COVID-19 Response-Driven Business Transformation for Pharmaceutical and Healthcare Industry
COVID-19 Response-Driven Business Transformation for Pharmaceutical and Healthcare Industry
The COVID-19 crisis is predominantly a humanitarian challenge, impacting the world at large. This eclectic reality is compass to the perspectives to follow. At the time of writing, the total COVID cases across the globe exceeded 5.8 million, increasing at an immoderate pace. Healthcare professionals and front-line public health workers are battling the pandemic, while putting their own lives at risk. With the pace of rise in COVID cases, healthcare infrastructure overload is the key area of concern.
Governments have implemented strict social distancing protocols among other restrictions to control the growth in the number of cases. Industries and Governments are trying to address the challenges arising from this crisis by working together to help the direct and indirect victims this crisis has brought about, all while hoping, coordinating and searching for an effective treatment or vaccine that would be the most crucial aspect of an impactful exit strategy. Until then, scaled-up testing is the go-to protocol for clarity on the intensity and spread of the virus. The pharmaceutical and healthcare industry, thereby becomes the most crucial stakeholder to pull the world out of the COVID-19 pandemic crisis.
The global pharmaceutical and healthcare industry stakeholders have been working together with unprecedented coordination leveraging global expertise towards phased testing of antibodies and vaccines. The World Economic Forum has reported that the phase-wise testing is progressing at an unparalleled pace, with process reports and feedbacks which earlier took weeks to process, getting responses over weekends and within days.
The pandemic has brought out the lack of resilience in the health systems around the globe and how they were inadequately prepared to deal with multiple health challenges. The most important lesson that it taught the world was to strengthen its healthcare infrastructure. The disruptive challenges caused by this pandemic for the pharmaceutical and healthcare industry are manifold.
On a global scale, pharmaceutical and medical product supply chains are struggling to keep pace with the rapid spread of the COVID-19 virus. The disruptive effects of COVID-19 have placed enormous strain on the global supply of medical products, increasing the risk of shortages.
Although production across various industries and regions in China have been gradually resuming since late February, global pharmaceutical and medical device manufacturers, who heavily source directly and indirectly from China, are now exposed to high risks in supply shortages over the upcoming months due to limited operational capacity. India, which is a major exporter of pharmaceutical products to several countries including the U.S, is also constrained. Despite the retraction on the ban imposed on the export of hydroxychloroquine, the biggest challenge remains to be the supply chain disruptions and operational difficulties in scaling up production to meet the surge in demand.
Tackling of the COVID-19 crisis is likely to have long term global implications. A country like India with certain strong basic aspects like a well-distributed pharmaceutical industry can develop its own models of nation-building by combining public health requirements and economy for national development.
Impact Assessment of COVID-19 on the Industry
With the number of cases on the rise, the society at large has been more attentive to the healthcare and pharmaceutical industries, which will impose both positive and negative consequences across different sub-sectors. In the short term, there will be a variety of impacts on pharma companies, healthcare institutions, pharmaceutical distribution and health insurance. In the medium to long-term, the impact on the healthcare and pharmaceutical industries are relatively positive. These are as follows:
- Societal awareness for disease control, prevention and healthcare will be enhanced
- The value of development and commercialization of medicine, vaccines and medical devices by the capital markets will be improved
- The Government will drive the development of hierarchical medical systems, strengthening the competence of community medicine and healthcare institutions
- Digitalization of the industry such as internet hospitals will speed up
- The demand for private health insurance will increase, currently dominated by social benefits or considered as a luxury due to lack of awareness or inadequately structured policy requirements
For pharma companies, positive impact areas include the increasing demand for drugs for prevention and treatment of coronavirus as well as the unprecedented attention for vaccine development.
The aspects of negative impact for the pharma industry are decreasing sales for chronic disease drugs and reduced promotion of innovative drugs with focus stagnant on COVID-19 response now. New drug launches could also be impacted because of this.
For healthcare providers and institutions, positive impact areas are accelerated development of Internet of Medical Things (IOMT), faster development of hierarchical medical systems with the involvement of community medical institutes. The prevalent demand surge could also provide opportunities for more third-party testing centres to alleviate resource shortage in healthcare institutions. The downside for healthcare institutes due to COVID-19 is the decrease in the footfall of patients seeking elective or non-emergency treatments. This could contribute to significant operational losses.
Within pharmaceutical distribution and retail, favourable winds are imminent for online pharmacies with increased user base for app based prescription drug delivery services.
Facing this emergency, Government regulators, medical educational universities and institutions along with pharmaceutical and medical device manufacturers have responded and adjusted quickly, by deconstructing the genomic sequence of the virus, manufacturing testing and diagnosis kits, screening for potential drugs as well as conducting clinical research, publishing procedures, policies and technical guidance on clinical trials. This is testimony to the impressive progress towards collaborative innovation in medicine.
Healthcare and Pharmaceutical Industry Impact in the Short term and Requirements in the Long-Term
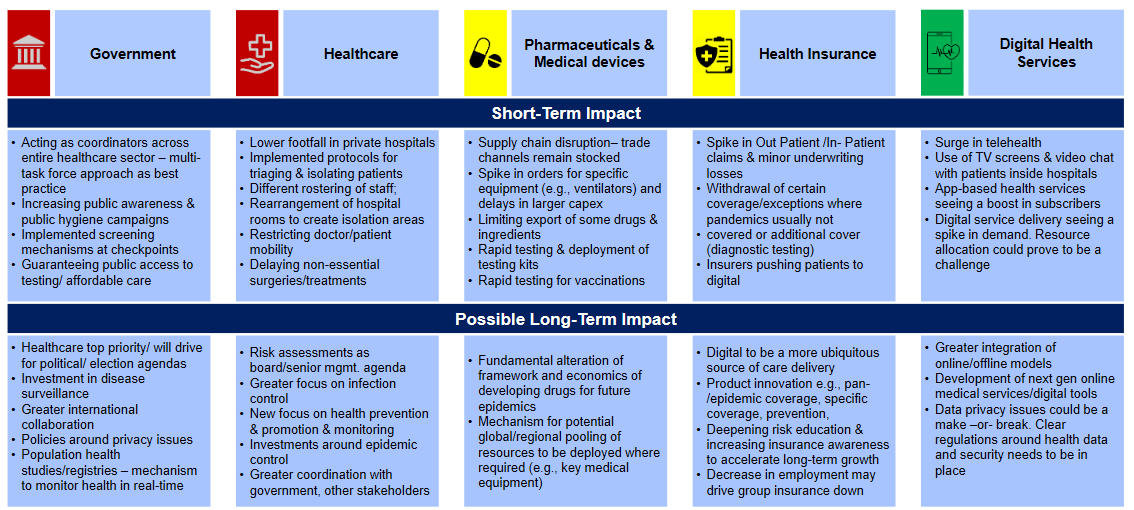
Strategy towards Business Transformation
Strategy towards Business TransformationA critical aspect of moving ahead and envisaging a conducive environment for business transformation is monitoring & establishing the ability to respond. The industry will have to bring about the resurgence together by building on the trust and confidence of both its community and allies. This can be facilitated by steps towards protecting their people & communicating frequently to ensure that the staff understands what to do and is aligned to the top management vision for recovery and transformation.
Organizations need to assess the extent of short-term exposure & key dependencies across the organization and rapidly set-up robust response team structures, governance & processes to ensure consistency and seal gaps and delay in communication. The Organization needs to establish decision authority for response team leadership & link to board of management
It is important to ensure the latest sources of facts & intelligence that can be disseminated to the rest of an organization on a daily basis while working with global & local health authorities, regulators and stakeholders to stay compliant to health advisories and standard protocols. There is the need to design immediate stabilization initiatives and a proportionate response mechanism while reaching out to peer organizations and sharing best practices within the industry.
Another important criteria to evaluate for business transformation is to understand the risk on the business and quantify exposures.
The organization should identify commercial and business exposures and implications in the second half of 2020 and beyond with respect to business operations reviews, supply chain and operational constraints. Wherever possible, quantify the potential implications on business and financials. This will then lead to developing the approach to track and report risks on impact including operational issues, strategic perspectives & financial resilience.
The organization should be able to develop a resiliency planwith focus on an updated demand planning, operating model & network optimization. Further, it would be beneficial to periodically stress test the adopted operational, strategic & financial resiliency measures.
The third key aspect to incorporate within the transformation plan is to consider the long-term commercial implications for the industry at large and the business entity in particular. The organizations would do well to revisit the existing business models to identify & capture opportunities to mitigate & differentiate business through products, integrating and coordinating between plant sites among others. If not committed yet, now is the time to invest in digital and build digital tools to augment offline process and enable large scale solutions
Going forward, greater coordination will be a requisite across the ecosystem while improve organizational coordination internally as well as with key health authorities and stakeholders to develop coordination plans inclusively. Businesses need to evaluate supply chain readiness to enhance supply chain measures.
Product Management Capability Evaluation Model for Pharmaceuticals and Medical Device Manufacturers
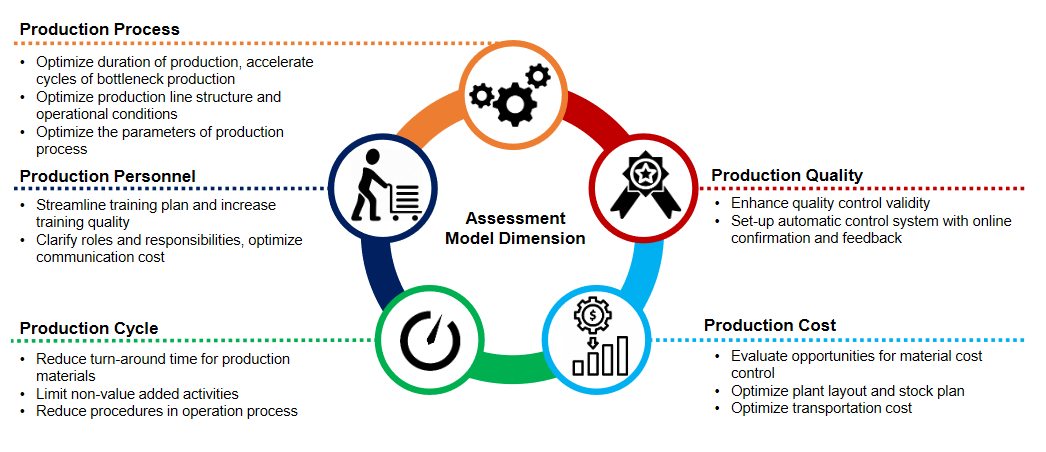
Industry Outlook –The Indian Perspective
The Indian generic pharmaceutical sector has always been a reliable source for cheap medicines for the world. But the outbreak has created several shortcomings for the industry because of shortage of Active Pharmaceutical Ingredient (API) supply. What the industry can immediately focus is on manufacturing that can be done with available raw materials and linking with the Indian supply chain instead of waiting for the revival of the global value chain.
The industry should form a consultation group of public health experts and medical personnel who can identify and prioritise requirements in the case of the outbreak of the epidemic and calibrate the production.
The urgent action required is to boost medical supplies. These include sanitizers, disinfectants, face masks, surgical gloves, protective gears for health personnel, test kits, infrared thermometers, scanners, ventilators, inhalers and so on. Some of these are high end technology but most require low level of technology only and can be easily manufactured. What could be done is that apart from the conventional pharmaceutical and medical equipment industry, other sectors of industry can join forces for ramped up manufacturing to meet the surging demand in the light of reduced demand for luxury products, thereby buffering some economic impact on themselves as well.
MSME is a sector that needs high focus in this endeavour of twining public health and economic development. This crisis situation could be made into an opportunity for MSMEs. They need to be given special incentives for producing low end technology items in medical and sanitary equipment like masks, gloves, cottons, etc. That will revive the stagnant sector.
The healthcare sector is facing a downturn compared to the pre-COVID state. Despite more than 60% share of bed capacity, the private healthcare sector’s financial performance was severely constrained. Stipulation of lockdown measures has further constrained the financial performance by March-end causing providers to make operational losses. If the situation persists, providers will face severe liquidity crisis with cash balance likely to cover operating losses only for 1-2 months. Operating losses are estimated to the tune of Rs 4,500 crore for a month and Rs 13,400 crore for a quarter if revenues are at 50% considering occupancy of 35%.
Liquidity infusion, indirect and direct tax benefits and fixed cost subsidies are among the key recommendations for financial relief to the sector through short-term interest free/ concessional interest loans and immediate release of complete dues locked with Central and State government authorities. It is indeed a relief that the government has taken steps to implement these measures.
It is necessary to develop the medical education models that are tuned to not only the public health needs but are also suited to the levels of pharmaceutical industry in the country, so that they become co-players in strategic national development.
Cushioning the COVID impact on our clients: Consocia’s value driven services providing dynamic solutions
As COVID-19 grew into a global crisis, Consocia realized the need to support industry colleagues in dealing with the biggest challenge faced ever in recent times that of business continuity. In response to the situation, we were swift in curating an in-house crack team comprising experts in research and insights; stakeholder database generation; content; government relations and public policy.
Consocia Advisory engaged Central and State Governments besides many Districts through strategic narrative backed by data to highlight the need of the hour in the fight against the deadly pandemic. We urged immediate orders to restore Client’s ability to manufacture, warehouse, transport and distribute the client’s essential products across the country.
Presently, Consocia is working with several enterprises for business continuity as well as crisis management. In the last few weeks, we have helped opening of plants and warehouses of the Indian entity of a global disinfectant company in 6 states including in Red zones as well as
Containment areas, besides that of a renowned lighting solutions company in two states (Haryana and Karnataka) already while they are now looking for our assistance in three more states.
Within a few days of being on-boarded, through our 24×7 support, we were able to secure not only policy interventions for manufacturing but also for warehousing, logistics and distribution as well as access to staff & workers. In the process, we were able to assure the Central and State Government stakeholders that all due precautions are being taken to prevent and contain COVID 19. We even helped with internal SOPs for transportation and staff movement.
We are helping the apex body representing the Shopping Malls across India against the debilitating impact the Coronavirus pandemic has had on them. On behalf of SCAI, Consocia has crafted several interventions to draw the attention of the stakeholders and policy-makers on the plight of the industry and reinforcing reasons for Malls to be considered for resuming operations in a staggered manner, for the post-lockdown phase. At the same time, Consocia is working with the empowered Group of Ministers and Committees for COVID-19 response as well as the RBI seeking urgent financial stimulus for the sector and amplifying the initiatives through media engagement from time to time.
The upcoming editions of the dynamics of business transformation white paper series will focus on specific industries with strategies and outcome driven solutions to positively impact business outlook for business recovery and continuity in the COVID-adjusted world.
COVID-19 is a long battle for the industry. As your trusted well-wisher, our team is available to support you during these uncertain times in the areas of business continuity planning, public affairs, public policy and government relations. Contact us: reachus@consociaadvisory.com
